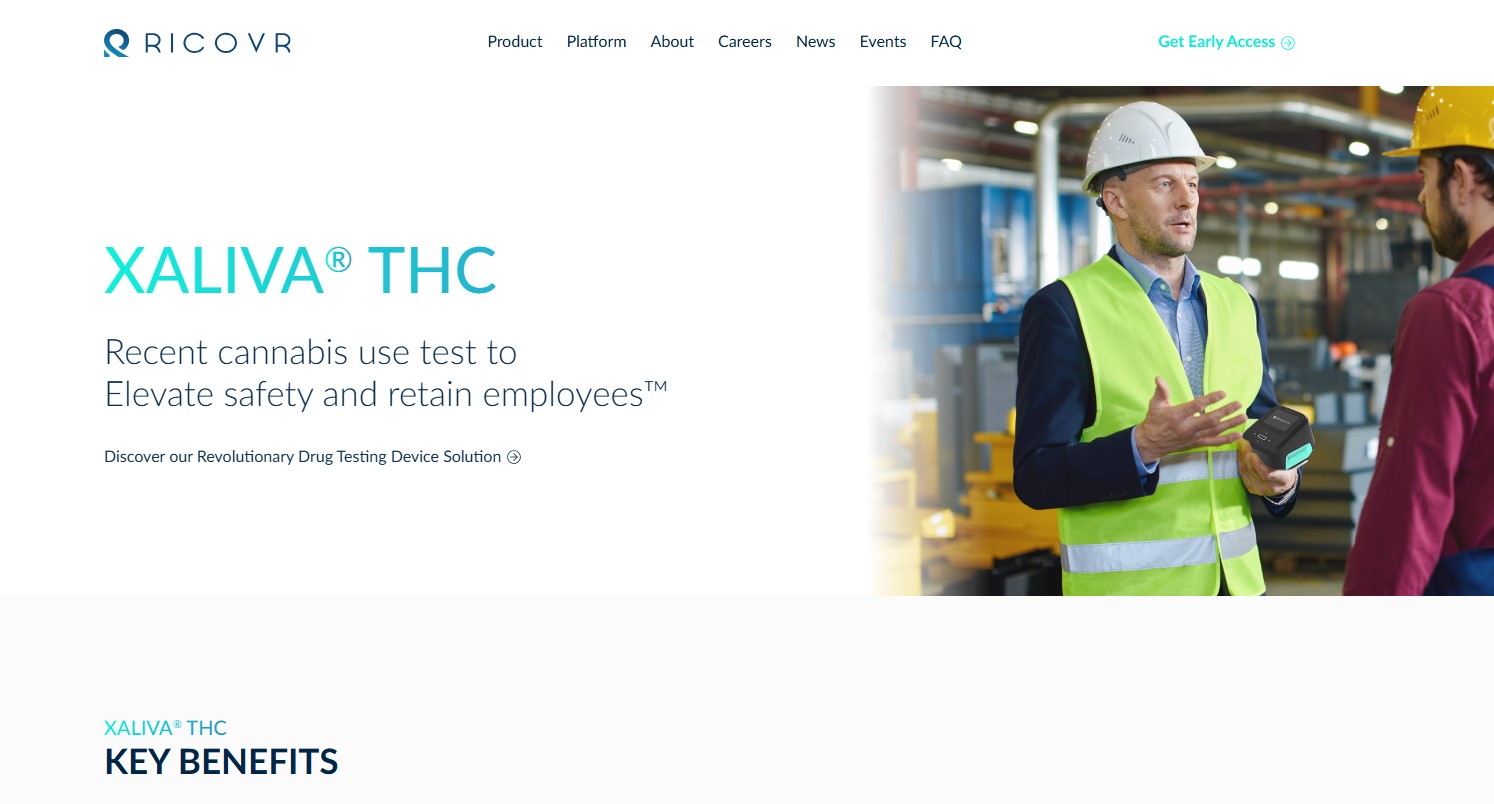
Ricovr Healthcare Wins NIH Award to Advance XALIVA®

PRINCETON, N.J., July 24, 2024 /PRNewswire-PRWeb/ — Ricovr Healthcare is proud to announce that it has been awarded a competitive Small Business Innovation Research grant (SBIR) from the National Institute on Drug Abuse (NIDA) at the National Institutes of Health (NIH) for the development of its groundbreaking cannabis testing platform, XALIVA®.
This innovative device aims to revolutionize drug screening and enhance public safety by providing accurate THC quantification in saliva using its automated point-of-care platform.
The NIH grant will support Ricovr in creating an effective, scalable, and accurate device that addresses the growing need for reliable drug screening solutions. XALIVA® strives to be a game-changer in the industry, offering precise and immediate results for cannabis detection.
Dr. Joseph Seimetz, Director of Research and Development at Ricovr Healthcare, will serve as the Principal Investigator for this project. Dr. Seimetz remarked, “We are thrilled to receive this NIH SBIR grant, which will enable us to advance our technology and bring a much-needed solution to the market. We aim to provide an easy-to-use, accurate, and reliable device that enhances public safety and addresses the challenges of on-site drug testing.”
Dr. Himanshu Bhatia, CEO and founder of Ricovr Healthcare, will serve as the Co-Investigator. Dr. Bhatia commented, “We are extremely proud to have secured this grant. This further validates our technology and underscores the importance of our mission. With the support of the NIH, we are confident that XALIVA® can set a new standard in drug screening and help ensure a safer environment for all.”
Ricovr Healthcare’s grant award from the NIH will accelerate the development of the XALIVA® platform. It will be a significant milestone in the company’s efforts to advance point-of-care testing across the healthcare technology sector. As the demand for a reliable and scalable THC testing solution increases, the market potential for XALIVA® expands, positioning Ricovr at the forefront of this critical industry.
Research reported in this press release was supported by the National Institute on Drug Abuse of the National Institutes of Health under award number 1R43DA059318. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
For more information about Ricovr Healthcare, please visit XALIVA® or contact: [email protected]
About Ricovr Healthcare:
Ricovr Healthcare is a leading innovator in point-of-care diagnostics, committed to enhancing public health and safety through its advanced optical detection technology. The Company’s flagship product, XALIVA® THC, represents the next generation of cannabis testing, providing accurate and immediate results for THC quantification in saliva. The Company’s product pipeline includes rapid point-of-care tests for THC, drug screening, reproductive health, and infectious diseases. The Company boasts an excellent management team of scientists and engineers, a distinguished advisory board, and excellent academic and industry partners around the globe who are all committed to improving diagnostics.