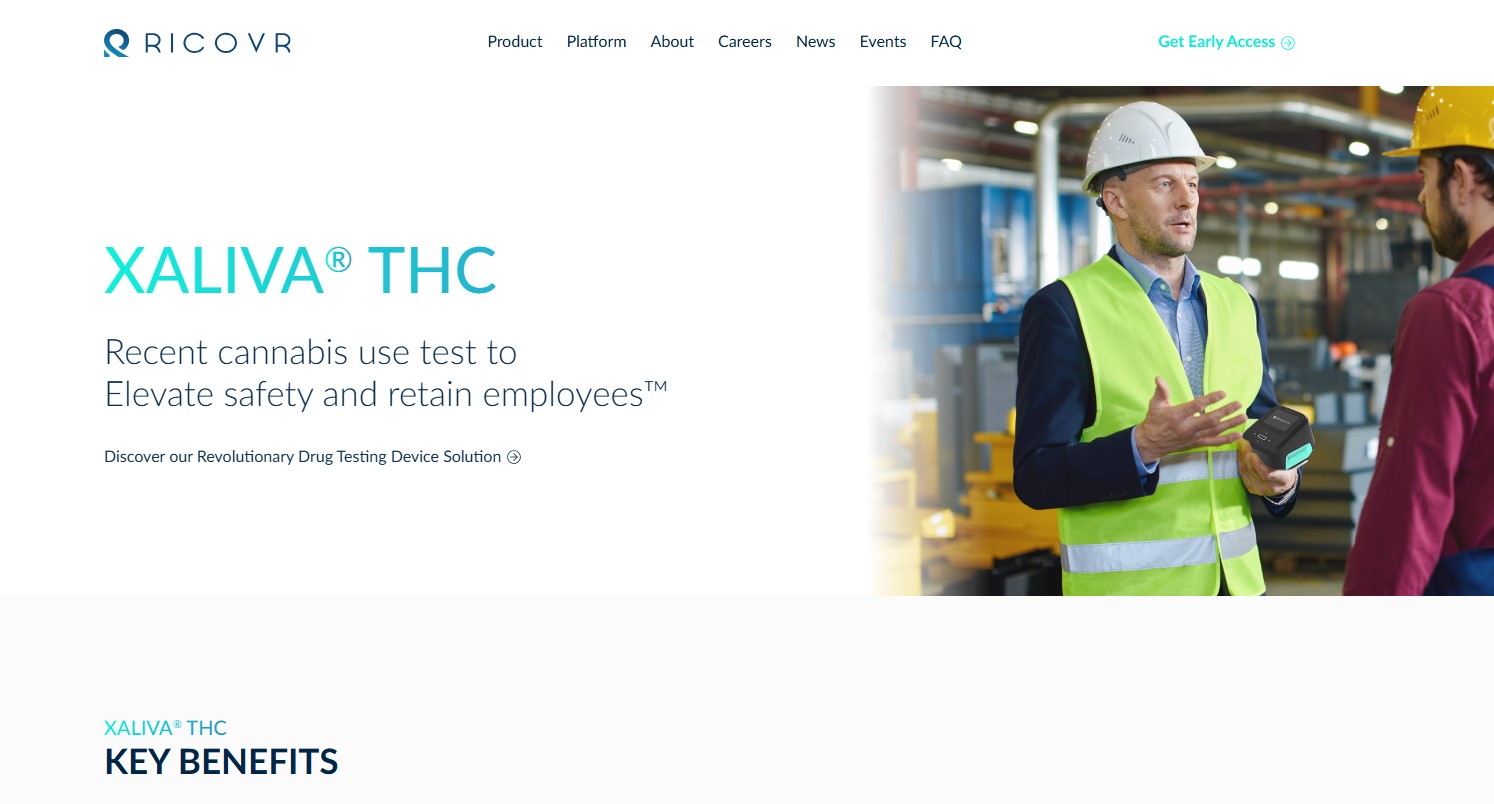
Looking for a QA/RA Lead with Project Management Skills
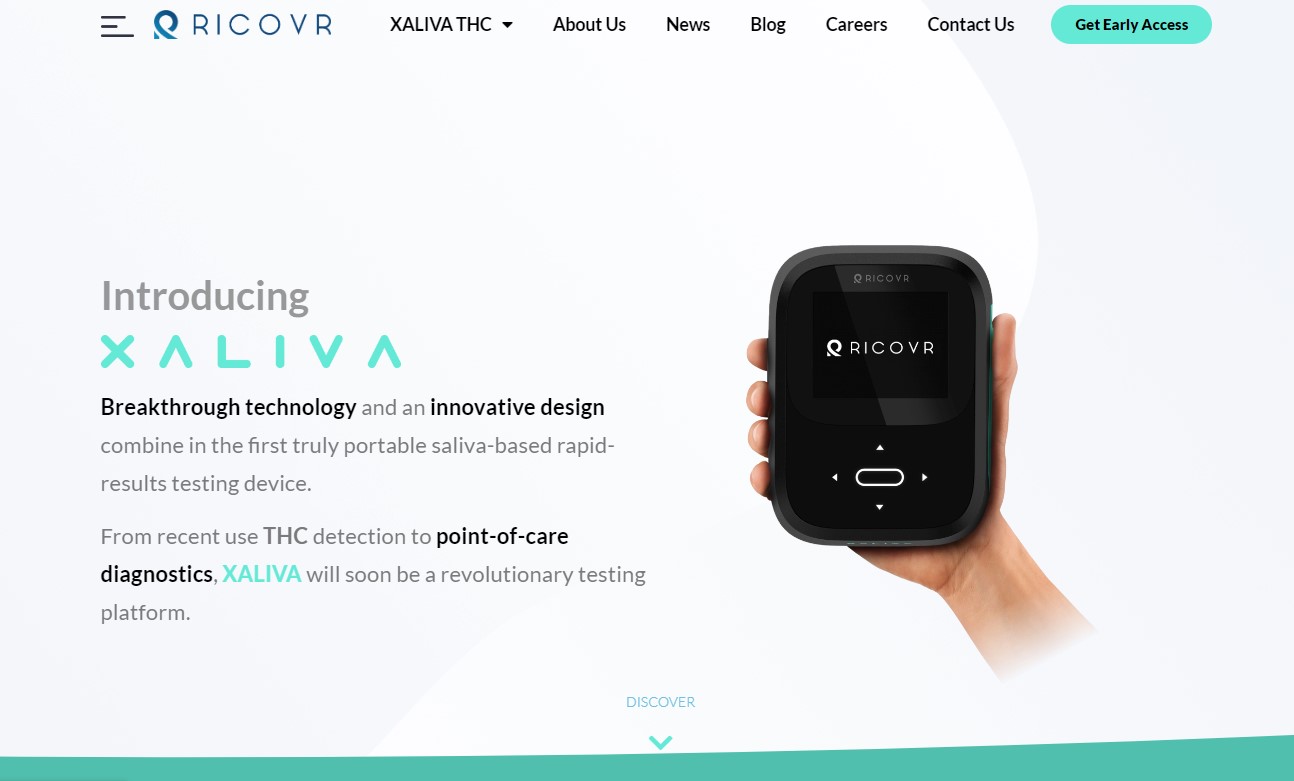
RICOVR is at an inflection point in bringing this technology to market. We are seeking a qualified and experienced Quality Assurance and Regulatory Affairs Lead with robust Project Management skills to join our team. This role is essential to guide the team in organizing and tracking work effectively and ensuring that all regulatory aspects of the project are compliant with relevant standards. This is an exceptional opportunity for a talented professional to contribute to the growth and success of our innovative organization.
Key Responsibilities:
Provide Guidance on Best Practices:
Offer insights and direction on the regulatory and quality aspects of the organization. Remain aware of new or updated regulations, laws, standards and other official enactments that may apply to the company.
Set up Part Numbering System and QMS:
Implement a part numbering system and Quality Management System internally or utilizing software to enhance operational efficiency.
Develop Regulatory Submission Plan:
Construct a detailed regulatory submission plan ensuring comprehensive and compliant documentation. Assist in the submission of registration applications. May assist in the preparation of IDE, 510(k), PMA, CE Mark, and other related regulatory filings. Chart pathways to FDA approval for law enforcement, DOT, and federal drug program use, and for CLIA submission.
Manage components Regulatory Approval and Sourcing:
Oversee regulatory approval and manage sourcing to ensure quality and compliance for accessories.
Obtain Appropriate IRBs:
Secure necessary Institutional Review Board approvals to uphold ethical standards for user testings and clinical trials.
Vendor Management and Supply Chain:
Manage vendors and supply chain effectively to ensure timely and cost-effective project completion.
Quality Management:
Offer insights and create a plan for managing the quality of reagents, product testing, stability, and labeling to ensure superior product quality.
Qualifications:
- A Bachelor’s of Science Degree in mechanical engineering, industrial engineering, life science, or other related discipline
- 3-5 years in a similar position responsible for in vitro diagnostics or medical device quality engineering duties.
- Regular practice of QA/RA skills and activities.
- Seasoned Project Manager / Change Agent that can effectively manage multiple projects at once.
- Demonstrated experience leading or participating in a successful in vitro diagnostic or medical device product launch.
- Comprehensive knowledge of regulatory standards and quality management systems.
- Strong written and verbal communication abilities with the ability to work closely with engineering and scientific teams to execute successful product launches
Compensation:
- Attractive salary and benefits
- Employee stock options
- Excellent working environment
Be a part of a team that is dedicated to enhancing the quality and efficiency of healthcare. Join us and make a significant impact in the industry, with opportunities for career growth and development.